MISSION AND PURPOSE
It has become increasingly apparent that inappropriate inflammatory and ineffective immune responses are at the center of many chronic disease states. Direct systemic modulation of these critical pathways however can have severe negative consequences as these are central pathways affecting multiple organ systems.
Vicapsys is focused on leveraging natural pathways in a localized manner in order to achieve beneficial therapies without systemic negative consequences. At the core of Vicapsys technology is a pleiotropic cytokine, CXCL12 that can be optimally formulated to achieve therapeutic outcomes in different indications.
Vicapsys is currently focused on curing Type 1 diabetes through long-term survival of transplanted islet cells using a formulation that inhibits development of the foreign body response and immune protection. Vicapsys is also leveraging its core technology to address the consequences of diabetes (diabetic foot ulcers) and the consequences of localized fibrosis (abdominal adhesions).
History
Company History and Highlights
- Platform technology exclusively licensed from Massachusetts General Hospital in 2013
- Broad portfolio (8 patent families) of issued and pending applications covering multiple indications/applications of the platform
- Platform is broadly divided into cellular and non-cellular product lines
- Cellular applications initially targeted to treatment of Diabetes Mellitus Type 1 through pancreatic islet cell transplantation
- Feasibility and long-term maintenance of transplanted human islet cells established in non human primate models
- Other indications include treatment of diabetic foot ulcers and peritoneal adhesions
Management
Board of Directors
LEVERAGING THE BODY’S NATURAL MECHANISMS
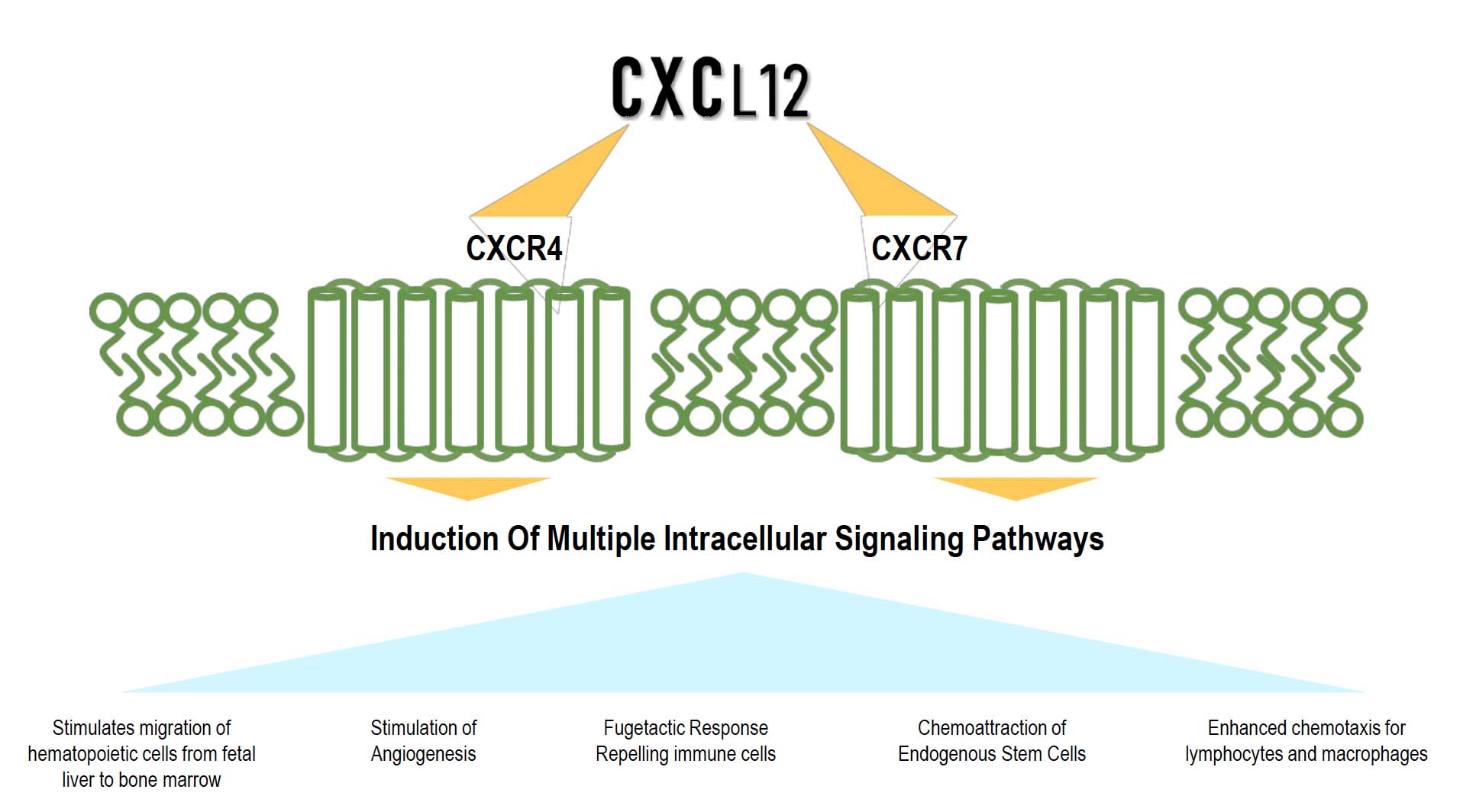
Multiple activities of CXCL12 for different applications
Key CXCL12 Discoveries
- Repulses Effector T cells and recruits Regulatory T cells to create an immune-privileged environment
- Permits and promotes the survival of transplanted β-Islet cells
- Promotes Anti-inflammatory and subsequently anti-fibrotic processes preventing post-surgical adhesions
- Promotes angiogenesis and recruits endogenous stem cells for the promotion of wound healing
Schematic of the CXCL12 Cellular Actions
Two Product Lines
CXCL12 Protects Insulin Producing Cells
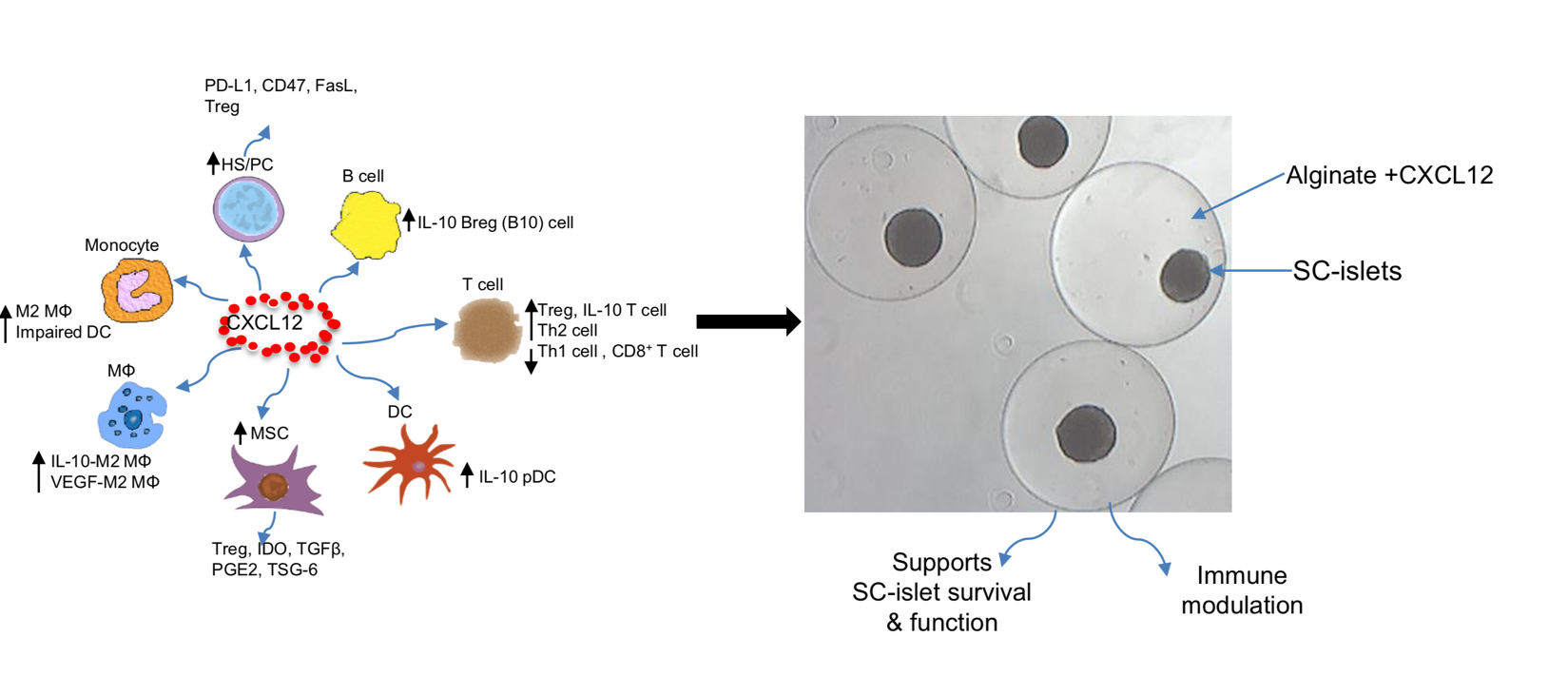
CXCL12-BASED THERAPIES IN DEVELOPMENT
ViCapsyn™
DIABETES TREATMENT BY β ISLET STEM CELL TRANSPLANTATION
Allogenic Human Stem Cells differentiated into β-cells to produce Insulin
- Transplanted in a matrix of an alginate containing CXCL12
- Rodent and NHP results indicate cell survival and glucose modulating function up to 6 months posttransplantation
ViBrin™
TO ACCELERATE HEALING OF DIABETIC FOOT ULCERS (DFUs)
- CXCL12 complexed into a bioabsorbable polymer and applied directly to the DFU following debridement surgery
- Inhibit inflammation, promote stem cell recruitment, angiogenesis to accelerate ulcer healing
ViBrin™
INHIBITING ADHESIONS POST-PERITONEAL SURGERY
- CXCL12 formulated in a bioabsorbable, moldable polymer applied to the incision site
- Inhibit inflammation and block formation of adhesions
THERAPEUTIC APPLICATIONS
ViCapsyn™ Treatment of Type 1 Diabetes
Diabetes Market Scope
- The global treatment market for type 1 diabetes will expand from $6.6b in 2013 to an estimated $13.6b by 2023.
- From 2000-2015 diabetes costs in the U.S. nearly doubled, from $12.05 to $23.35 million.
- The number of adults living with diabetes has almost quadrupled since 1980.
- Diabetes is one of the leading causes of death in the world, and in 2012, was the direct cause of 1.5 million deaths.
Cell Transplantation Program For Type 1 Diabetes
- Funded by and developed in partnership with the JDRF
- Diabetes Allotransplant Experiments in Non-Human Primates (NHP) are On-Going
- Identifying the optimal β-stem cell partner for clinical studies
Alters Innate Immune Response To Omentally-implanted Islets In Animal Models
Concept and Direction
- Incorporation of the fugetactic molecule, CXCL12 into an alginate polymer to create an immune-protected environment for transplanted islet cells
- CXCL12 alginate formulation helps maintain survival of transplanted islet cells for functionality for extended periods
- Human islet stem cells as an autologous or allogeneic cell source produce insulin in response to plasma glucose levels
ViCapsys Data
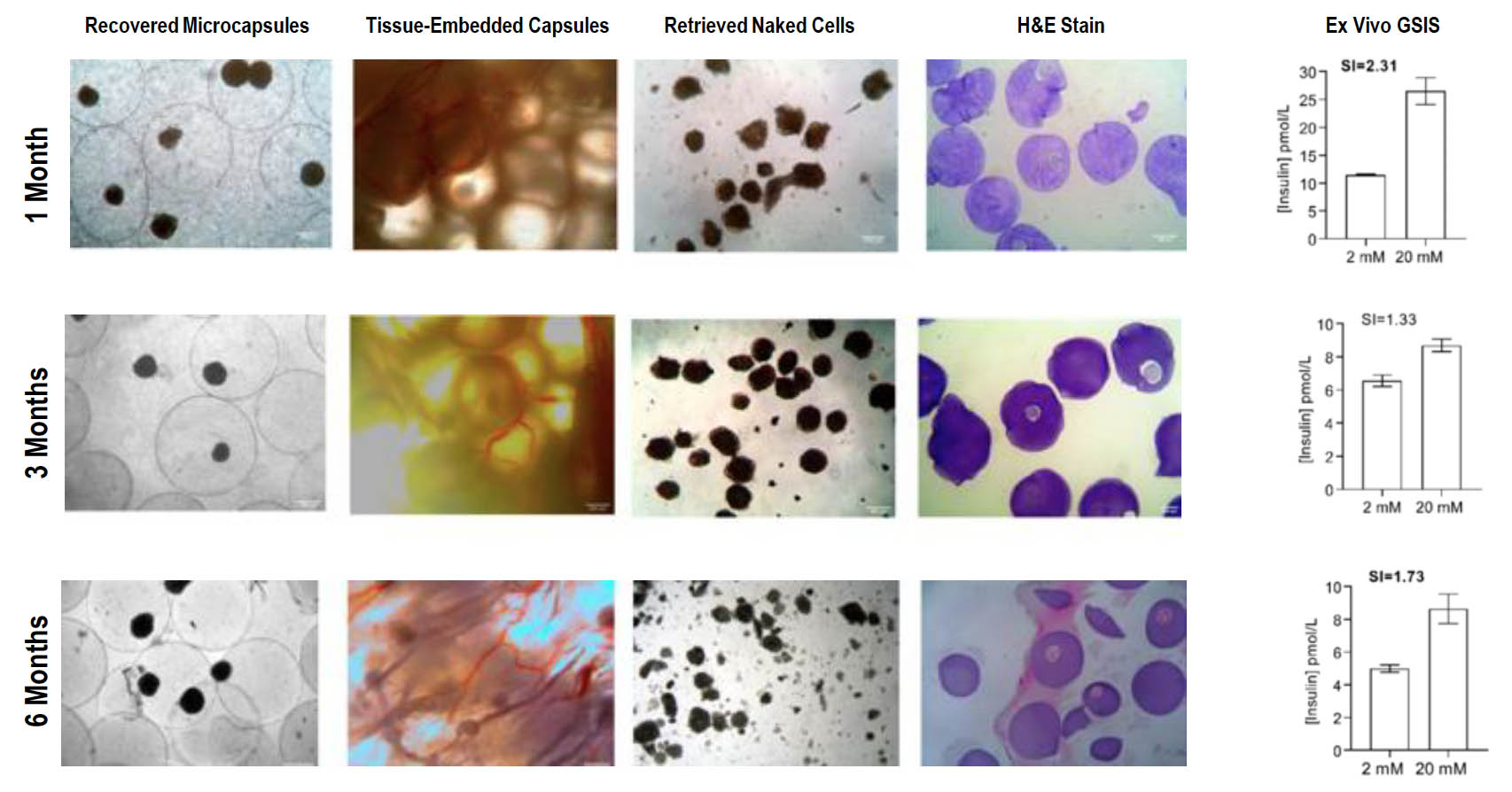
- Data from mouse models and non-human primate studies have demonstrated that islet cells encapsulated in alginate formulations containing CXCL12 are protected from fibrotic overgrowth and foreign body response.
Majority of Microencapsulated SC-Islets Are Fibrosis Free and Maintain Glucose-Responsiveness Up to 6 Months
VICAPSYN™ Eliminates Immune Rejection of Transplanted Islets
- Approximately 100,000 encapsulated islet equivalents placed into separate non-human primates
- Implants were surgically evaluated at 90 days
- Periodic blood tests were used to evaluate systemic immune responses
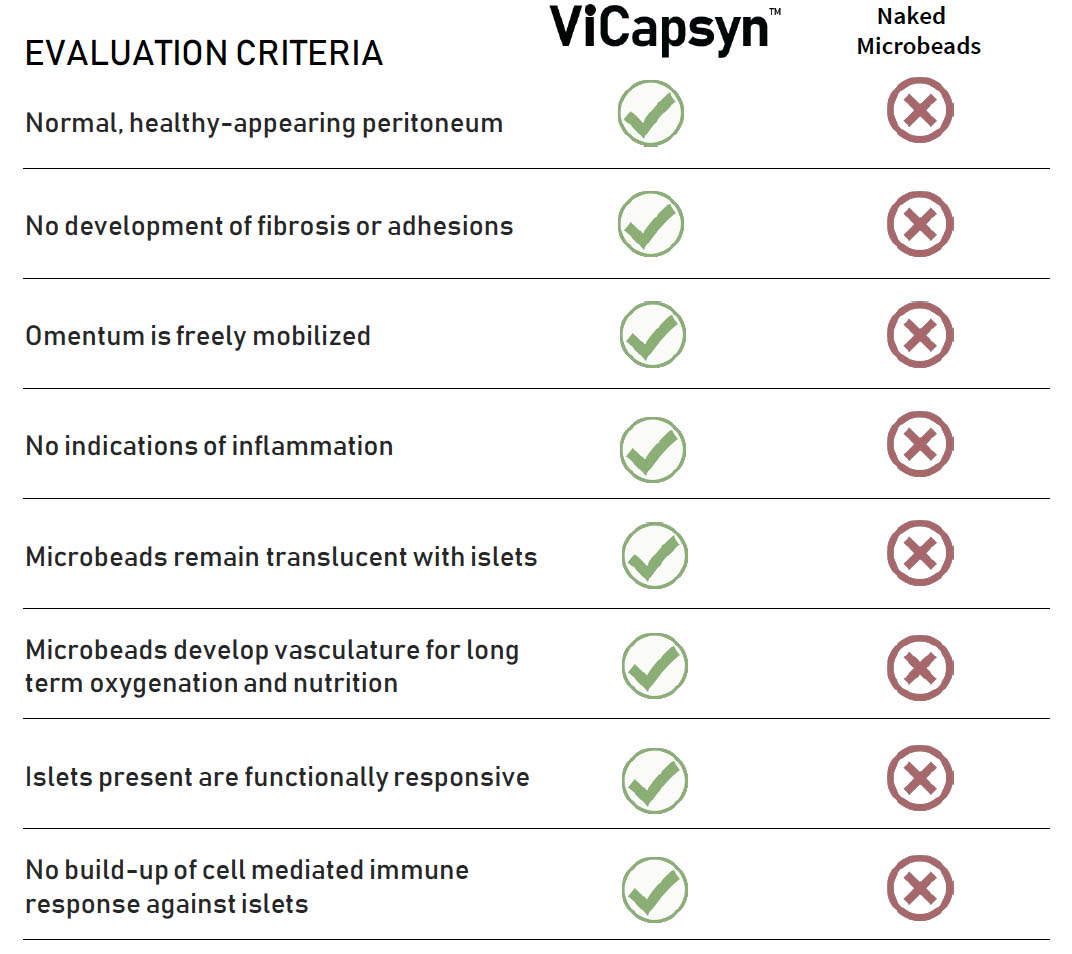
Transplantation Of Islet Cells: Latest For Primate Data Summary
- Microencapsulation of human stem-cell (SC) derived islets with CXCL12 minimizes foreign body reaction and supports long term (6 months) function without immunosuppression in a primate
- Non-diabetic primates transplanted with SC derived islets maintain glycemic control suggesting a glucose regulated function
- SC islets maintained expression of all major endocrine hormones and glucose responsiveness over 6 months
- No observed hematology or biochemical dysfunction
Latest Data
- Preliminary demonstration of efficacy of stem cell derived beta cell transplantation in the context of healthy primates now out to six months post transplantation. Studies with diabetic primates ongoing
ViBrin™ Treatment of Diabetic Foot Ulcers & Adhesions
Diabetic Foot Ulcer (DFU) Market Scope
- Between 1.0 – 3.5 million diabetic patients in the United States have a history of foot ulceration
Adhesions Market Scope
- Demand for adhesion barriers surgeries poised to grow at a CAGR of 9.2% between 2016 and 2024
- Estimated global market of greater than US$ $USD2.7 Billion by 2020
- North America market is expected to be US$ 1.6 Billion during 2016 to 2026
Non-Cellular Program Addresses Two Conditions
TREATMENT OF DIABETIC FOOT ULCERS DFUs
are a significant financial burden to health care systems and significantly impact the Quality of Life indices for patients and their families
PREVENTION OF POST-PERITONEAL SURGERY ADHESIONS
An unmet medical need with more than 10 million patients at risk in the US and 25 million patients globally
A Solution to Accelerate Healing of DFUs and Prevent Recurrence
What are Diabetic Foot Ulcers Diabetic foot ulcers?
(DFUs) are a serious complication of diabetes that result in significant morbidity and mortality.
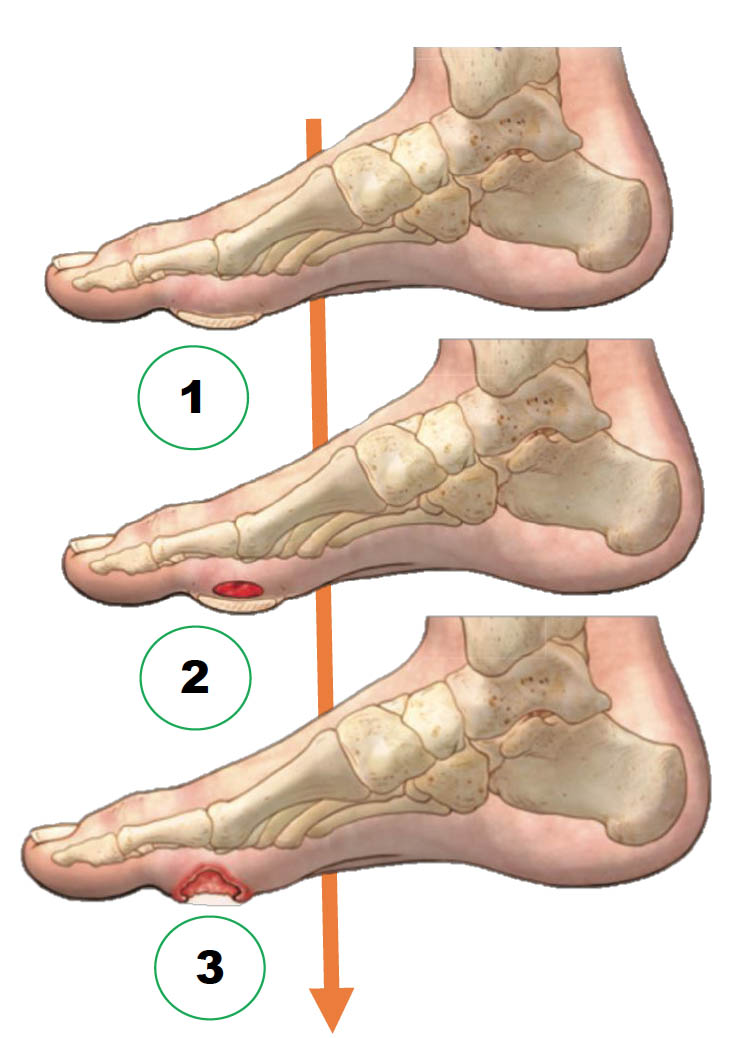
The Common Pathway of Diabetic Foot Ulcer Occurrence and Recurrence
- Repetitive external or minor trauma
- Trauma leads to a subcutaneous hemorrhage
- Hemorrhage results in DFU
Current Status of DFU Treatment
- The standard practices in DFU management include surgical debridement, dressings to facilitate a moist wound environment and exudate control, wound off-loading, vascular assessment, and infection and glycemic control
- DFUs are responsible for more than 80.0% of all diabetes-related lower-leg amputations and occur in around 15.0% of diabetic patients
- Even after successful treatment, 40% of patients recur after 1 year and 60% of patients recur after 3 years
- Mortality rates associated with development of a DFU are estimated to be 5% in the first 12 months, and 5-year morality rates have been estimated at 42%
The Vicapsys Concept and Direction of DFU Treatment and Care
How we leverage our CKCL12 platform into a therapy:
- CXCL12 is complexed into a bioabsorbable polymer and applied directly to the DFU following debridement surgery
- Our compound Inhibits inflammation, promotes stem cell recruitment, and angiogenesis to accelerate ulcer healing
- Evaluation of single agent and combinations in validated animal models
Accelerated DFU Healing in Hyperglycemic Mice Treated with Bacteria-Producing CXCL12
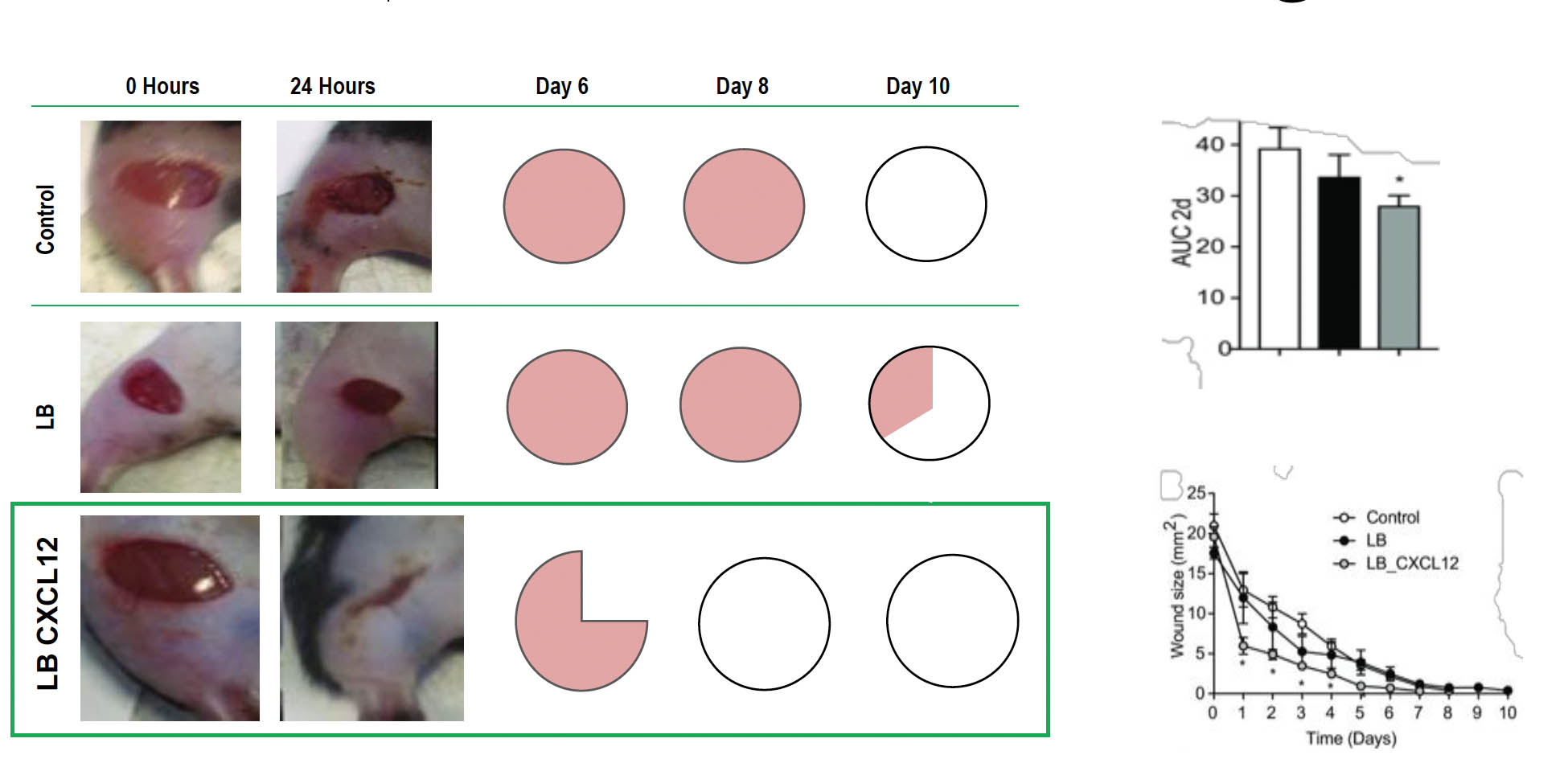
Accelerated wound healing by treatment with CXCL12-producing L. reuteri. (A) Wounds receiving no treatment (control), treatment with control L. reuteri (LB), and CXCL12-expressing L. reuteri (LB_CXCL12) at the time of wound induction (0 h) and at 24 h. Wound sizes (B) and accumulated wound surface area at 2 d (C). (D) Fractions of wounds healed by different treatments at days 6, 8, and 10 (control, n = 4; LB, n = 3; LB_CXCL12, n = 8; *P < 0.05).
A Solution To Prevent Post-Peritoneal Surgery Adhesions
What are Adhesions
An adhesion is a band of scar tissue that binds two parts of tissue or organs together. The tissue develops when the body’s repair mechanisms respond to any tissue disturbance, such as surgery, infection, trauma, or radiation. Adhesions occur after up to 97%of abdominal interventions causing chronic pain, infertility, and intestinal obstruction.
CONCEPT AND DIRECTION
- Incorporation of CXCL12 into a biodegradable polymer as a solid polymerized substance or as a solution that polymerizes upon contact with tissue
- Application of the CCL12-Polymer at the site of surgical intervention
- The presence of CXCL12inhibits the inflammatory and immune system attack at the surgical incision, allowing the tissue to repair without the formation of adhesions
- Mouse models and non-human primate studies have demonstrated that microbeads containing CXCL12 protect the surrounding tissue from fibrotic overgrowth and adhesions
VIBRIN™ Blocks Early Inflammatory Response: Prevention Of Adhesions
Post-Operative Adhesion Prevention
The Main in adhesion Prevention Include:
- Improvement of surgical techniques
- Limitation of the intra-abdominal organ’s trauma
- Application of adjuvant agents to reduce the formation of adhesions
- Adjuvant agents are typically an Adhesion-Barrier material inserted into the incision site and frequently have little or no effect
- Improvement of surgical techniques
- Limitation of the intra-abdominal organ’s trauma
- Application of adjuvant agents to reduce the formation of adhesions
- Adjuvant agents are typically an Adhesion-Barrier material inserted into the incision site and frequently have little or no effect
- Adhesions are a very common and potentially debilitating consequence following surgeries
- In 2009 there were 6.1 million digestive system surgeries and 1.1 million urogenital surgeries in the US. (www.stanfordhealthcare.org)
- As many as 97% of all abdominal and pelvic surgeries result in the formation of adhesions which may present clinically as chronic and severe pain, infertility in women and in 3-13% of the cases mortality
- The cost to the healthcare system and to the quality of life for the patient is significant
- Current preventive therapies are physical adhesion barriers and as evidenced by the high incidence rate of adhesions, they are not effective or difficult to use in the operating room
- There is no approved pharmacological intervention to prevent adhesions
Adhesion Barriers Currently On The Market
- Polytetrafluoroethylene (Preclude®): largely used in gynecological operations WL Gore and Associates.
- Polylactide membrane (Surgiwrap®) MAST Biosurgery AG, – a clear film attached to the site of incision.
- 4% Icodextrin solution (Adept®) (Baxter)
- Hyalobarrier® gel and Hyalobarrier gel endo (Anika Therapeutics and NordicPharma, ) .They are composed of highly purified, auto cross-linked polymers of hyaluronic acid (ACP®).
- Seprafilm® (Sanofi, Paris, France) Seprafilm adhesion barrier is a medical device for adhesion prevention for patients undergoing abdominal or pelvic laparotomy
- Interceed (Barrier, Absorbable, Adhesion: Ethicon, Johnson & Johnson) Interceed(TC7) is a fabric composed of oxidized, regenerated cellulose that was designed to reduce the formation of postsurgical adhesions. 1989
BUT THERE ARE NO LEADERS IN EFFICACY
As many as 97% of all abdominal and pelvic surgeries result in the formation of adhesions which may present clinically as chronic and severe pain, infertility in women and in 3-13% of the cases mortality.
NEWS & EVENTS
Publications
ViCapsys Life Sciences Appoints Three New Board Members
ViCapsys Life Sciences today announced the appointment of Mr. Richard Rosenblum, Dr. Colleen Delaney, and Mr. Charles Farrahar to the Company's Board of Directors, effective September 22, 2022...
ViCapsys Life Sciences Appoints Federico Pier as Permanent Chief Executive Officer
ViCapsys Life Sciences, Inc., (OTC:VICP) (“ViCapsys” or the “Company”) announced the appointment of Federico Pier as Chief Executive Officer of ViCapsys, effective February 15, 2022. Mr. Pier had been serving as interim President & CEO since August 2020.
Preliminary Studies of the Impact of CXCL12 on the Foreign Body Reaction to Pancreatic Islets Microencapsulated in Alginate in Nonhuman Primates
We previously demonstrated that the incorporation of the chemokine CXCL12 into alginate microbeads supported long-term survival of microencapsulated auto-, allo-, and xenogeneic islets in murine models of diabetes without systemic immune suppression. The purpose of this study was to test whether CXCL12 could abrogate foreign body responses (FBRs)...
Long-term Survival of Transplanted Allogeneic Cells Engineered to Express a T Cell Chemorepellent
Alloantigen specific T cells have been shown to be required for allograft rejection. The chemokine, stromal cell derived factor-1 (SDF-1) at high concentration, has been shown to act as a T-cell chemorepellent and abrogate T-cell infiltration into a site of antigen challenge in vivo via a mechanism termed fugetaxis or chemorepulsion. We postulated that this mechanism could be exploited therapeutically and that allogeneic cells engineered to express a chemorepellent protein would not be rejected...
Alginate Encapsulant Incorporating CXCL12 Supports Long-Term Allo- and Xenoislet Transplantation Without Systemic Immune Suppression
Islet transplantation represents a potentially curative approach for individuals with Type I Diabetes....
Alginate‐microencapsulation of human stem cell–derived β cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression
Pancreatic β‐cell replacement by islet transplantation for the treatment of type 1 diabetes (T1D) is currently limited by donor tissue scarcity and the requirement for lifelong immunosuppression...
Events
Check here for events we will be attending…


